Ferrous hydroxychlorides hibbingite (γ-Fe2(OH)3Cl) and parahibbingite (β-Fe2(OH)3Cl) as a 1 concealed sink of Cl and H2O in ultrabasic and granitic systems
Peter Koděra, Juraj Majzlan, Kilian Pollok, Stefan Kiefer, František Šimko, Eva Scholtzová, Jarmila Luptáková a Grant Cawthorn
American Mineralogist 2022, Vol. 7, 826-841 – Link
Abstract
Ferrous hydroxychlorides are geochemically important but less recognized mineral species due to their extreme sensitivity to oxidation and hydration in contact with air {typically they convert to akaganéite [Fe3+(O,OH,Cl)]}. Only the γ-form was previously known as the orthorhombic mineral hibbingite, associated with altered mafic intrusive rocks. In this study, we describe the β-polymorph of Fe2(OH)3Cl as a new mineral parahibbingite that was found in pyroxenite from the Karee platinum mine in the Bushveld Complex, South Africa. The two minerals were distinguished by a combination of Raman spectroscopy and FIB-SEM-TEM analytical techniques (TEM-EDX and TEM-SAED). They can be easily recognized by their distinct Raman spectra. Parahibbingite has two very strong vibration bands at ~3550 and 3560 cm–1, accompanied by much weaker bands at ~124 and 160 cm−1, while the Raman spectrum of hibbingite has a sharp, strong band at 3450 cm−1 and two moderate bands at 199 and 385 cm−1.
Parahibbingite was found as fine-grained reaction rims at the contact of orthopyroxene phenocrysts and talc inside a drill core. It has a trigonal space group [R3m, a = 6.94(5) Å; c = 14.5(2) Å], with an empirical formula (Fe2+1.98Mn2+0.01Fe1.982+Mn0.012+Ca0.01)(OH)3.08Cl0.92. The origin of this mineral in the Bushveld Complex is most likely related to a late hydrothermal alteration of pyroxenite. Hibbingite forms as an abundant daughter mineral hosted by fluid inclusions and salt melt inclusions in hydrothermal quartz associated with granitic systems during cooling under reducing conditions. Such inclusions are common in Au-porphyry mineralization worldwide, such as the Biely Vrch (Slovakia) deposit studied in detail in this work. The lattice parameters obtained by TEM-SAED are a = 6.30 Å, b = 7.12 Å, and c = 9.89 Å.
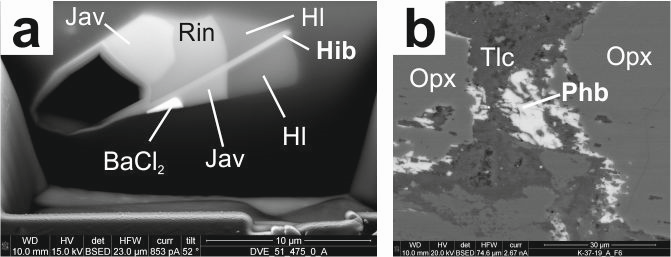
Hibbingite was recognized as the only phase that carries “water” (as a hydroxyl group) in otherwise water-free, salt melt inclusions. Furthermore, both hibbingite and parahibbingite should be considered as reservoirs for Cl and H2O in large volumes of altered basic and ultrabasic rocks. They can transport volatiles to shallow levels of subduction zones. Alternatively, their dissolution can fuel remobilization, transport, and deposition of sulfidic ores in saline fluids. Their detection, however, is difficult because of their sensitivity to oxidizing atmospheres. For example, in natural outcrops exposed to air, they may vanish, thus distorting estimates of their abundance and role in many processes that involve mineral-derived volatiles.


